November 28, 2025
Oral CDK2 Inhibitor is a clinically translatable drug for cisplatin-induced hearing loss
Cisplatin, a mainstay cancer drug, causes irreversible hearing loss in up to 60% of patients, creating a major unmet need. With ∼500,000 U.S. patients receiving platinum and other platinum-based therapies annually, preventing ototoxicity is critical.
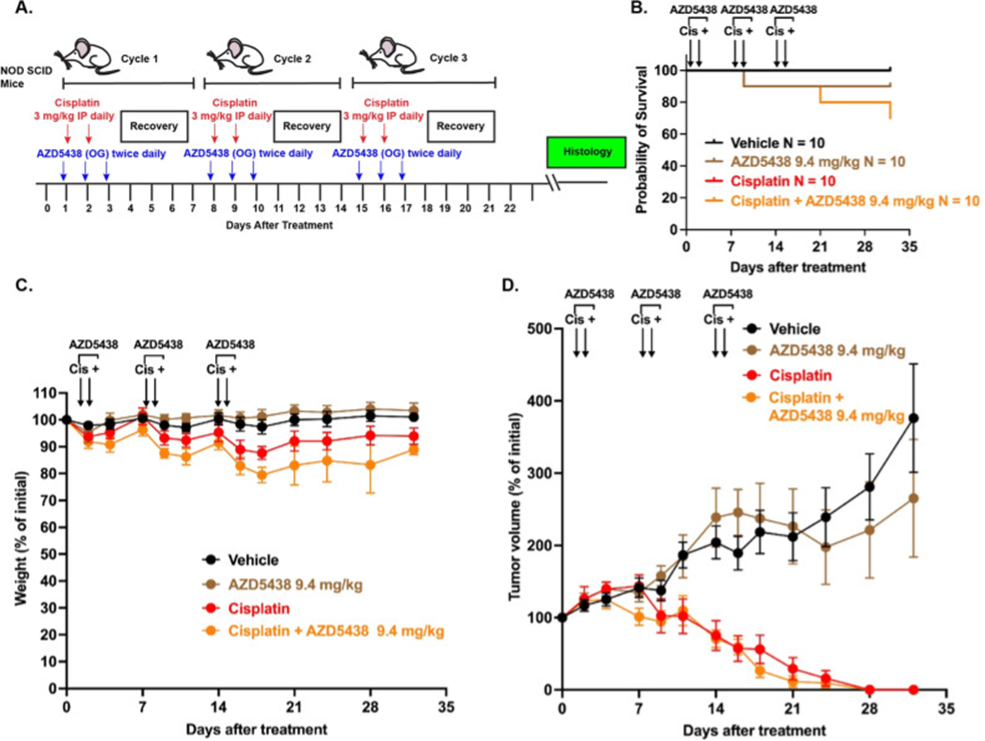
In a collaborative study led by Dr. Tal Teitz, an Associate Professor at the Scintillon Research Institute, with Drs. Jian Zuo, Yunlong Huang and colleagues from Ting Therapeutics, Inc, a selective CDK2 inhibitor was evaluated as a potential otoprotectant. Building on animal studies performed at the Scintillon Research Institute, pharmacokinetic comparisons defined a human-equivalent tolerated dose in mice showing cross-species consistency, within which AZD5438 robustly protected against cisplatin-induced ototoxicity in an established multi-cisplatin clinically relevant mouse model. The study also addresses a key translational barrier—whether AZD5438 interferes with cisplatin’s efficacy—and found that otoprotective doses do not compromise its anti-tumor effect in a testicular xenograft tumor model, removing a major hurdle to clinical translation (Figure above). The study published in the journal Neoplasia https://doi.org/10.1016/j.neo.2025.101250 supports first-in-human trials of AZD5438 as an otoprotectant and highlights its potential as a supportive cancer therapy that preserves hearing without compromising efficacy. In addition, previous findings that AZD5438 synergizes with dabrafenib (an FDA-approved BRAF inhibitor) suggest that both compounds may act on complementary stress response or apoptotic signaling pathways. These findings support further clinical development of AZD5438 and provide a compelling rationale for dose-acceleration studies in Phase I/II clinical trials targeting cisplatin-induced hearing loss in cancer patients. In the future, the study’s results may enable testing in clinical trials an increase of the total dose of cisplatin used for cancer treatment as AZD5438 can also protect from cisplatin-induced acute kidney injury as was shown before by this group https://doi.org/10.1681/ASN.0000000000000261.
This new work was supported in part by NIH grants R43/R44DC018463 awarded to Ting Therapeutics and R01DC018850 awarded to Tal Teitz.
Support the Future of Science
Your generosity can help accelerate this groundbreaking work from concept to cure. Tax-deductible donations directly support vital research on hearing loss associated with cancer treatment and aging at the Teitz Lab. Major contributions may also allow for program growth, investment in advanced equipment, and the creation of lasting legacies, including named research fellowships, endowed chairs, or lab spaces. Please consider donating today.