Scintillon News
Pedersen Lab awarded CDMRP grant from DoD
March 19, 2024 08:30 AM
The Pedersen lab has been awarded a $5M Congressionally Directed Medical Research Programs Consortium grant from the Department of Defense (TP230352) titled “The Role of Genetic Risk Factors and Immune Response on NVU Function in post-TBI Cognitive Impairment.”
Recent advances and challenges of HIV therapeutics
November 17, 2023 08:30 AM
Dr. Amber Khan and Dr. Nandagopal Paneerselvam in Prof. Brian Lawson's lab review challenges and success of broadly neutralizing antibodies (bNAbs) and antiretroviral therapy for HIV treatment.
A novel genetically encoded tool manipulates redox metabolism with sub-cellular precision and can be used to interrogate the role of reductive stress in various pathologies.
November 13, 2023 08:30 AM
Scintillon Institute scientists validated a soluble transhydrogenase from E. coli (EcSTH) as a genetically encoded tool to induce NADH reductive stress in both mammalian cells and mouse liver. Characterization of the reductive stress using this tool revealed unique transcriptional and metabolic signatures. This new technology will be extremely useful for future studies aimed at identifying the root causes of multiple diseases or conditions linked to mitochondrial metabolism or an imbalance of redox cofactor NAD.
The Scintillon Institute is Awarded CIRM Grant to Assess Engineered Hematopoietic Stem Cells as a Viable HIV Treatment Option
November 29, 2022 11:47 AM
Associate Professor Brian Lawson, Ph.D. Antiretroviral therapy currently on the market is highly effective in the treatment of HIV, lowering viral loads and inhibiting disease progression; the problem is that these drugs do not affect dormant HIV hidden in host immune cells and, over time, are associated with significant toxicity. Therefore, how do we completely eliminate HIV, active and latent, from an infected person? One exciting approach to achieve that goal is the use of chimeric antigen receptor (CAR) T cell immunotherapy, which gained broad recognition as a new treatment modality for certain blood cancers.
SURE Program 2022
September 19, 2022 10:22 AM
"It's a wrap!" - Scintillon's summer high school student research program excites young minds and comes to an end for 2022. Every year, Scintillon’s research faculty perform exciting biomedical research pushing for a cure of many major disease conditions, including neuroscience, immunology, virology, and aging-associated diseases. Towards these ends, the faculty has to secure new grants and new philanthropy to resource these efforts. In addition to these difficult challenges, every summer the faculty also joins together and donates a significant amount of their time and effort to teach and host a growing number of very highly motivated local San Diego high school students in Scintillon’s research laboratories for an intensive 4-week training program.
This protein can help us think, or it can shred our cells
February 07, 2022 8:10 AM
San Diego – A new study reveals that a protein long known to play a role in communication between cells in the brain is also capable of obliterating cells if left unchecked because of its penchant for twisting and puncturing the cell membranes. The protein — known as complexin — if left alone is so toxic it can shred cells. Yet, in the brain, a suite of controls makes sure the protein plays nice and helps neurons communicate by aiding in the release of neurotransmitters. The findings are published Feb. 7 in Nature Structural and Molecular Biology.
International Conference on Three-dimensional Cryo-EM Image Analysis
January 19, 2022 7:26 AM
The 4th International Conference on Three-dimensional Cryo-EM Image Analysis will be held March 9-12, 2022, at Granlibakken Conference Center, Lake Tahoe, California. The 2022 conference builds on our successful previous events on the same theme in 2014, 2016 and 2018. This year the conference is organized by Scintillon Professors Dorit Hanein and Niels Volkmann. The goal of this series of meetings is for technical discussions of state-of-the-art image analysis approaches and algorithm developments to tackle challenging biological problems and to identify current limitations in the field.
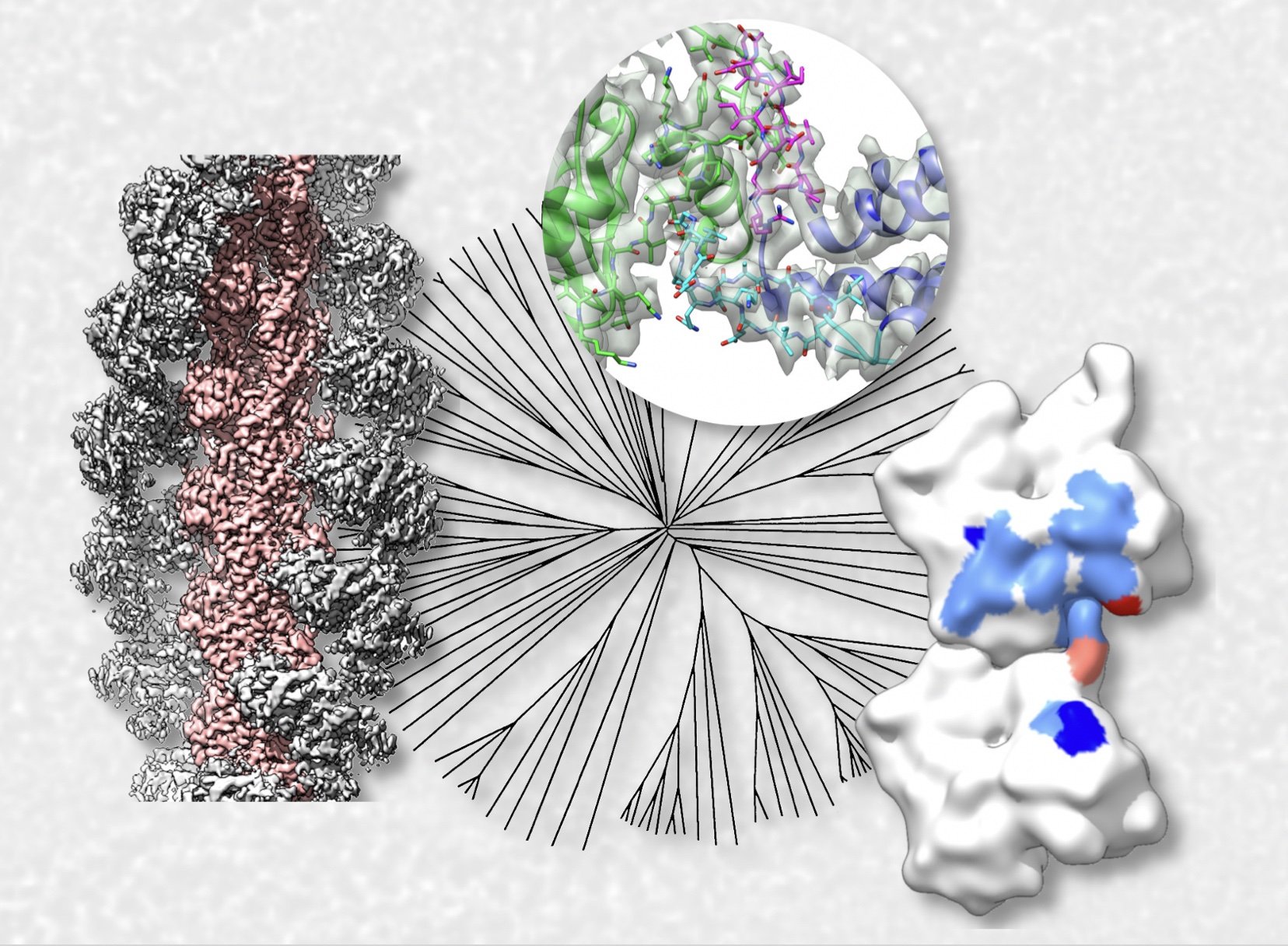
Recent advances in structural biology research by Scintillon researchers
January 19, 2022 7:26 AM
Rapid tool for cell nanoarchitecture integrity assessment Detailed three-dimensional contextual information of molecular processes is often necessary to understand these processes well enough to develop efficient drug-targeting and disease intervention strategies in all medical fields. We developed a tool that significantly accelerates studies providing such information (Gaietta et al., 2021 Journal of Structural Biology). Structural basis of aE-catenin–F-actin catch bond behavior A better understanding of how cell-cell contacts are maintained or broken is essential for unraveling the detailed mechanism of cancer progression.
A brain network that curbs the urge to eat
November 17, 2021 9:18 AM
Scintillon Institute researchers uncover an evolutionarily conserved brain region that puts a “brake” on food intake which may advance therapeutics for obesity and related disorders with excessive eating such as Prader-Willi syndrome. Do you ever wonder why you stop eating even when there are appealing foods available? The cerebellum compares hunger state with after-eating nutritional status in the gut to regulate dopamine reward signals in the striatum to control meal size.
Scintillon Institute Investigator Awarded NIH R35 Early-Career Grant
August 30, 2021 3:56 PM
Metabolism or the set of life-sustaining chemical reactions defines the very wellbeing of humans. But can we better understand how cellular metabolism goes awry in various human diseases? The National Institute of General Medical Sciences just granted Scintillon’s Dr. Valentin Cracan $2.4 million to find out more! The National Institute of General Medical Sciences (NIGMS) has recognized Dr. Valentin Cracan as one of the nation’s highly talented and promising scientists to receive a Maximizing Investigators’ Research Award for Early-Stage Investigators (R35 MIRA-ESI). This grant provides about $2.4 million over five years to support the ongoing work at the Cracan’s lab that has received a number of NIH grants already since it was established about 3 years ago. We spoke with Valentin as we were interested in his research in the context of his new, special grant.
Does science save lives during this pandemic?
July 21, 2021 10:47 AM
- Did science save lives during this pandemic that we are still in? - Are Biomedical and Bioengineering Sciences interesting and important subjects? “Yes!” many of today’s high school students will say that without hesitation. But is science research a field where you want to build a career? Many students may still want to say “Yes…” but maybe blindly. Scintillon Institute, a premium San Diego research institution for nonprofit purposes, has built its now famous Scintillon “SURE” (SUmmer REsearch) program to help top-performing high school students to find out for themselves whether conducting scientific research and becoming a career scientist is for them.
A New Twist in the Tale of Experimental Immunotherapies for Parkinson’s Disease
April 23, 2021 10:11 AM
A new study co-led by Scintillon Institute’s Associate Professor, Dr. Rajesh Ambasudhan, and Adjunct Professor Dr. Stuart Lipton (also a practicing Neurologist at UC San Diego) shows that certain immunotherapy approaches for Parkinson’s disease (PD) may cause harmful neuroinflammation by activating microglia (brain’s immune cells) and that this adverse effect could offset therapeutic benefits elicited by the antibody treatment. The study appeared in the April 13, 2021 issue of the Proceedings of the National Academy of Science of the United States of America.
Celebrating Scintillon wins in early 2021
April 15, 2021 10:21 AM